Chlorofluorocarbons (CFCs) are nontoxic, nonflammable chemicals containing atoms of carbon, chlorine, and fluorine. They are used in the manufacture of aerosol sprays, blowing agents for foams and packing materials, as solvents, and as refrigerants. Numbers in the names of CFCs indicate the number of atoms of carbon, hydrogen, fluorine, and chlorine.
After a series of fatal accidents in the 1920s with leaking refrigerators CFCs replaced the toxic gases ammonia (NH3), methyl chloride (CH3Cl), and sulfur dioxide (SO2), used as refrigerants in the late 1800s and early 1900s. Three US corporations - Frigidaire, General Motors, and Du Pont - collaborated in the search for a less toxic replacement and synthesized CFCs in 1928. The invention of home air-conditioning in 1932 expanded the use of CFCs. After World War II, CFCs were used as propellants for bug sprays, paints, hair conditioners, and other health care products. During the late 1950s and early 1960s the introduction of air-conditioning in cars spread its use further.
CFCs are inert in the lower atmosphere but undergo significant reaction in the upper atmosphere or stratosphere. In the mid-1970s it became clear that the CFCs could be a major source of inorganic chlorine in the stratosphere following their photolytic decomposition by UV radiation and that some of the released chlorine would become active in destroying ozone in the stratosphere.
Ozone (O3) is an isotope of oxygen normally found in the atmosphere. In the lower atmosphere ozone concentrations are usually low; increased ozone concentrations cause health problems for humans and other organisms.
In the upper atmosphere ozone is found in relatively high concentrations and acts as a filter for ultraviolet (UV) rays. UV radiation can cause skin cancer, so the presence of ozone in the upper atmosphere is essential to protect organisms. A loss of stratospheric ozone allows more harmful UV radiation to reach the Earth's surface. Chlorine released from CFCs destroys ozone in catalytic reactions where 100,000 molecules of ozone can be destroyed per chlorine atom.
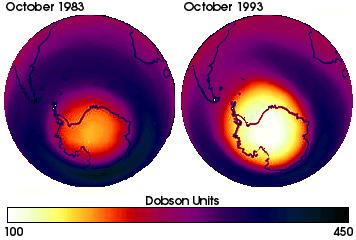
By 1985 scientists noticed that significant loss of ozone occurred in every spring and that the loss was getting worse each following year. The destruction of upper atmosphere ozone is particularly effective in very low temperatures. The most prominent consequence of CFCs in the atmosphere is therefore an "ozone hole" over Antarctica. This hole increased in size over the last decades:

(A Dobson unit is used to express ozone content in the upper atmosphere. It gives the thickness of the layer formed if all ozone is compressed under conditions of 0°C and 1 atmosphere pressure. 1 Dobson Unit is defined to be 0.01 mm thickness.)
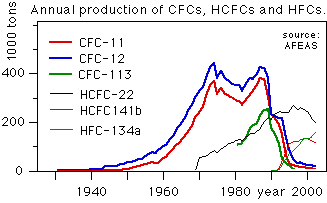
In 1987 the Montreal Protocol to Reduce Substances that Deplete the Ozone Layer agreed to reduce 1986 production levels of these compounds by 50% before the year 2000. An amendment of 1990 called for the elimination of production by the year 2000. The treaty was initially signed by 27 nations and now has 148 signatory members. The manufacture of CFCs chemicals ended for the most part before 1996.
Two classes of halocarbon substitutes, the hydrochlorofluorocarbons (HCFCs) and the hydrofluorocarbons (HFCs), have been developed. The HCFCs still contain chlorine which makes it possible for them to destroy ozone. The Copenhagen amendment calls for their production to be eliminated by the year 2030.
The science that became the basis for the Montreal Protocol resulted in the awarding of the 1995 Nobel Prize for Chemistry jointly to S. Rowland, M. Molina and Paul Crutzen for their work on the formation and decomposition of ozone.
Alternative Fluorocarbons Environmental Acceptability Study (AFEAS) (2004) Production and sales of fluorocarbons. http://www.afeas.org/production_and_sales.html (accessed 12 October 2004).
Elkins, J. W. (1999) Chlorofluorocarbons (CFCs). In: Alexander, D. E. and R. W. Fairbridge (eds.) The Chapman & Hall Encyclopedia of Environmental Science. Kluwer Academic, Boston, MA, 78 - 80.