Michael Faraday
Physicist and chemist, b. 22 September 1791 (Newington, Surrey, England), d. 25 August 1867 (Hampton Court, Surrey).
 Michael Faraday was the son of a blacksmith who had come to South London in search of work. His father was often too ill to work steadily, and his mother had a hard life to feed the family. The Faradays belonged to a Christian sect, and young Michael's only education was the Sunday school where he learnt the essentials of reading and writing.
Michael Faraday was the son of a blacksmith who had come to South London in search of work. His father was often too ill to work steadily, and his mother had a hard life to feed the family. The Faradays belonged to a Christian sect, and young Michael's only education was the Sunday school where he learnt the essentials of reading and writing.
Michael began to work as a delivery boy for a book dealer and bookbinder and became his apprentice at the age of 14. He took the opportunity to read the books he had to deliver, and the article on electricity in the Encyclopædia Britannica stimulated him to perform some basic experiments.
On one occasion Faraday was given a ticket to a lecture of Sir Humphrey Davy, chemist at the Royal Institution of Great Britain in London. He took copious notes, worked them out in detail at home, bound them at work and sent them to Davy with a humble request for employment. There was no vacancy, and Faraday had to continue with bookbinding.
In 1812 Davy remembered Faraday when one of his assistants was dismissed after a brawl. During the next eight years Faraday learnt everything in chemistry that could be learnt at the time. Davy was one of the key chemists to exploit the new chemical ideas of Lavoisier, and as his assistant Faraday became familiar with theories about atoms, molecules and forces exerted by them and acting between them. By 1820 the Royal Institution derived much revenue from Faraday's services as an expert in legal trials and as a consultant to industry.
After a few years of successful chemical research, during which he isolated benzene, improved the methods of steel production and produced a glass of very high refractive index for telescopes, Faraday turned to electricity and magnetism. in 1820 Hans-Christian Ørsted had published his paper that described how an electric current in a wire produces a magnetic field, and Ampère had shown that the magnetic force had to be symmetric around the wire, ie circular. Faraday concluded that this could be used to convert electrical energy into mechanical motion. The demonstration device he built was the first electrical motor.
In 1831, when he was involved with experiments in acoustics, he observed that if he forced a metal plate sprinkled with powder to vibrate he not only created patterns in the powder of the plate but also in the powder of a plate nearby. This acoustic induction led him to try the same effect on electromagnetism. It was to become his most famous experiment and showed that an electromotive force is generated in an electrical field if the magnetic field associated with it is changed.
Faraday then concentrated on the quantitative aspect of the phenomenon. He knew that a magnetic field of a magnet can be made visible by iron filings, which align along lines of force. He surmised that the force produced during induction was determined by the number of lines cut by the conductor during equal time intervals and concluded that a continuous current could be produced if the magnet was moved continuously. This led him to build the first dynamo.
When the question arose whether electricity generated by a rotating magnet is the same kind of electricity as that generated by an electric eel or that stored on a Voltaic battery, Faraday began a series of experiment to show that there is only one kind of electricity. This led to the formulation of Faraday's two laws of electrochemistry:
- The amount of a substance deposited on each electrode of an electrolytic cell is proportional to the quantity of electricity passed through the cell.
- The quantities of different elements deposited by a given amount of electricity are in the ratio of their chemical equivalent weights.
By 1839 Faraday had overworked himself to such an extent that his health was severely impaired. The period until 1845 was one of recuperation, with little science. In 1846 he returned to research and focussed on his belief that all forces were only manifestations of a single universal force and that different manifestations could be converted into each other. On a suggestion of William Thomson (Lord Kelvin) he experimented with magnetic fields created by electrical forces, which could be made much stronger than electrostatic ones. His experiments led him to write that space was filled with force fields and that energy was not localized in particles but distributed through space.
The last decade of Faraday's life was one of growing senility and increased public honours. Queen Victoria offered him a cottage and a knighthood; Faraday accepted the cottage but declined the knighthood. He was buried at Highgate Cemetery in London.
References
Williams, L. P. (1995) Michael Faraday, Encyclopaedia Britannica 15th ed.
Science Education Centre Soweto (2002) Historical Approach to Teaching of Science, http://www.sec.org.za/physics/phistor.html (accessed 1 June 2004)
Faraday's demonstration experiment
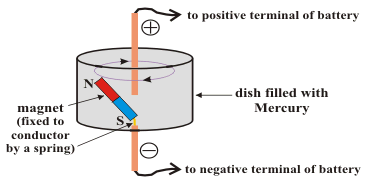
The magnet is loosely connected by a spring. When a current is sent through the mercury container the magnet rotates permanently around the axis of the container. (Science Education Centre, 2002)
home
 Michael Faraday was the son of a blacksmith who had come to South London in search of work. His father was often too ill to work steadily, and his mother had a hard life to feed the family. The Faradays belonged to a Christian sect, and young Michael's only education was the Sunday school where he learnt the essentials of reading and writing.
Michael Faraday was the son of a blacksmith who had come to South London in search of work. His father was often too ill to work steadily, and his mother had a hard life to feed the family. The Faradays belonged to a Christian sect, and young Michael's only education was the Sunday school where he learnt the essentials of reading and writing.